When your doctor talks about a biologic drug, it might sound like science fiction. These are medicines made from living cells-like tiny biological factories-that treat serious conditions like rheumatoid arthritis, Crohn’s disease, certain cancers, and diabetes. But now, you might hear about something called a biosimilar. Is it the same? Is it safe? And why does it cost less?
Biosimilars aren’t generics-here’s why that matters
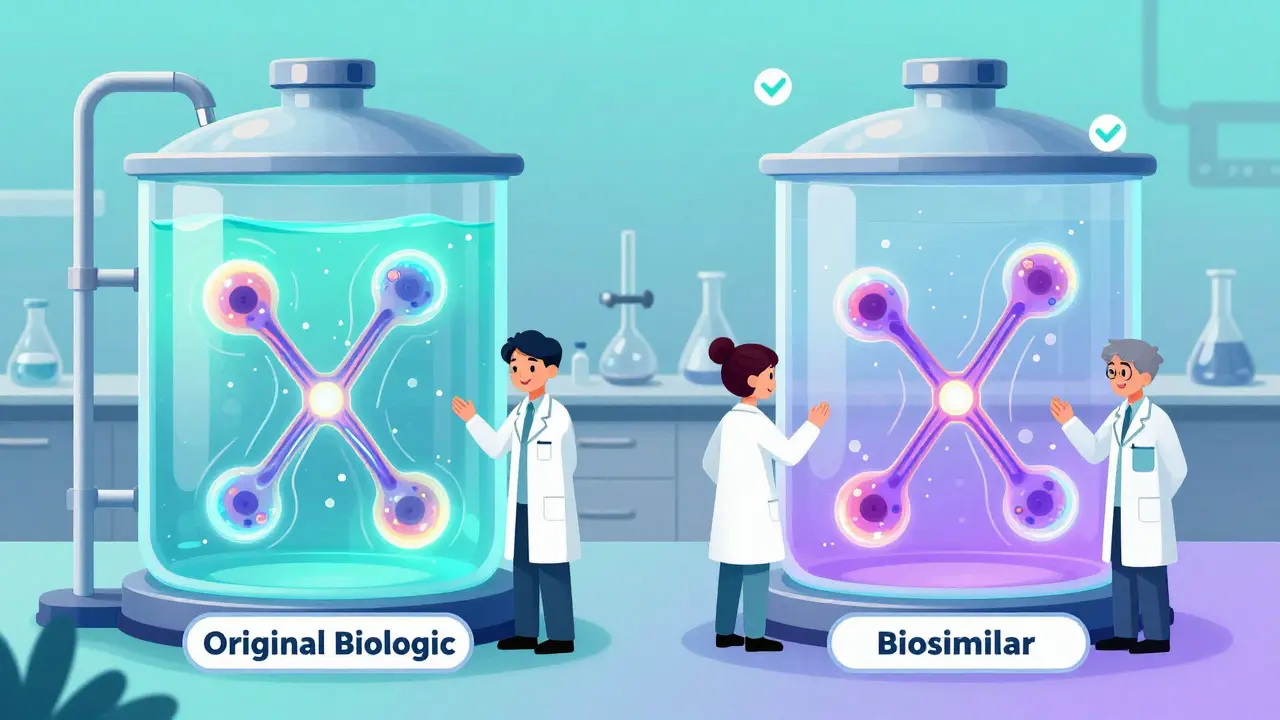
If you’ve taken a generic pill like ibuprofen or metformin, you know what generics are: exact chemical copies of brand-name drugs. They’re simple, small molecules made in a lab. Biosimilars? They’re completely different.Biosimilars are made from living cells-human, animal, or microbe-and are used to copy complex biologic drugs. Think of it like this: if a generic is a photocopy of a black-and-white document, a biosimilar is a hand-painted replica of a detailed oil painting. No two paintings are exactly the same, but if the colors, brushstrokes, and composition match closely enough, the result looks and feels identical.
The U.S. Food and Drug Administration (FDA) says a biosimilar must be highly similar to its reference biologic-with no meaningful differences in safety, strength, purity, or how well it works. That’s the key. It’s not an exact copy. It’s as close as science can get, given how complicated biological molecules are.
How are biosimilars made, and why is it so hard?
Biologic drugs are made using living systems-usually genetically modified cells grown in huge tanks. These cells produce proteins that target specific parts of the immune system or disease pathways. Even tiny changes in temperature, pH, or the type of cell used can change the final product.That’s why biosimilar makers don’t just reverse-engineer the original drug. They have to build their own cell lines, optimize their own production process, and purify the protein in a way that matches the original as closely as possible. Then, they run hundreds of tests-analyzing molecular structure, how the drug behaves in the body, and how safe it is in animals and humans.
For example, the biosimilar Renflexis (infliximab-abda), used for arthritis and Crohn’s, was tested in a clinical trial with over 500 patients. The results showed it worked just like the original drug, Remicade. No surprises. No hidden risks.
Biosimilars are just as safe and effective
You might worry: “If it’s not the same, is it risky?” The answer is no-when approved by the FDA, biosimilars are held to the same strict standards as the original biologic.The FDA doesn’t approve biosimilars lightly. They require:
- Extensive lab testing to prove the molecule is nearly identical
- Animal studies to check for toxicity
- Human clinical trials to prove it works the same way
- Monitoring of side effects and immune reactions
And the results? Over 15 years of real-world use in Europe-where biosimilars have been around longer-show no increase in side effects or loss of effectiveness compared to the original drugs. In the U.S., since the first biosimilar (Zarxio, a cancer support drug) was approved in 2015, thousands of patients have used them safely.
Organizations like the American Cancer Society and the Arthritis Foundation confirm: biosimilars behave the same way as their reference drugs. If your original biologic helped control your symptoms, the biosimilar will too.
What’s the difference between biosimilars and interchangeable biosimilars?
There’s a special category called “interchangeable” biosimilars. These are biosimilars that the FDA says can be swapped for the original drug without asking your doctor again-just like how pharmacies swap generic pills for brand names.The first one approved in the U.S. was Semglee, an interchangeable version of insulin glargine (Lantus), used for diabetes. That means if your prescription says “insulin glargine,” your pharmacist can give you Semglee without calling your doctor-unless you or your doctor objects.
Not all biosimilars are interchangeable yet. That’s because the FDA requires extra studies to prove you can switch back and forth between the original and the biosimilar without any impact on safety or effectiveness. But more are coming.
Will your insurance cover a biosimilar?
Yes-and often, they’ll push you toward it. Because biosimilars cost 15% to 30% less than the original biologic, insurance companies and Medicare often require you to try the biosimilar first before covering the more expensive version.That doesn’t mean you’re being treated like a guinea pig. It’s just smart economics. Lower costs mean more people can get the treatment they need. In fact, experts estimate biosimilars could save the U.S. healthcare system over $50 billion between 2017 and 2026.
Some patients worry: “If I switch, will my condition get worse?” Studies show that switching from a reference biologic to a biosimilar is safe. Patients with psoriasis, arthritis, and inflammatory bowel disease have been tracked for years after switching-and their outcomes stayed the same.
How do you know if you’re getting a biosimilar?
Biosimilars have different names than the original drugs. The generic name is the same, but with a four-letter suffix added at the end.For example:
- Original: Humira (adalimumab)
- Biosimilar: Ayuvie (adalimumab-afzb)
That suffix isn’t random-it’s there to help track the drug if any safety issues come up later. Your prescription will list the exact name. If you’re unsure, ask your pharmacist or doctor. They’ll know.

What conditions are treated with biosimilars?
Biosimilars are used for many serious, chronic conditions:- Arthritis (rheumatoid, psoriatic, ankylosing spondylitis)
- Inflammatory bowel disease (Crohn’s, ulcerative colitis)
- Certain cancers (breast, colon, lymphoma)
- Diabetes (insulin)
- Eye diseases (macular degeneration)
- Autoimmune disorders (lupus, multiple sclerosis)
More are being developed every year. In 2023, the FDA approved over 30 biosimilars for use in the U.S., and the global market is expected to grow to over $30 billion by 2028.
What should you do if your doctor suggests a biosimilar?
Talk to them. Ask:- “Is this biosimilar approved by the FDA?”
- “Has it been tested in people like me?”
- “Will it work the same way as my current drug?”
- “Can I switch back if I don’t feel right?”
Most doctors are confident in biosimilars. They’ve seen the data. They’ve watched patients do just as well on them as on the original.
But if you’re nervous, that’s okay. You don’t have to switch right away. You can ask for time to think about it. Or ask for a trial. Many patients start on a biosimilar and find their symptoms stay controlled-and their bills get smaller.
Bottom line: Biosimilars are a smart, safe option
Biosimilars aren’t cheaper because they’re lower quality. They’re cheaper because they don’t need to repeat the massive research costs of the original drug. The science is solid. The approval process is strict. The results are proven.If you’re on a biologic drug and your doctor offers a biosimilar, it’s not a downgrade. It’s an upgrade-same effectiveness, same safety, lower cost. And for many people, that means better access to life-changing treatment.
Always talk to your doctor before making any changes. But know this: biosimilars are here to stay-and they’re helping more people get the care they need.


Milla Masliy on 12 January 2026, AT 15:58 PM
Just had my rheumatoid arthritis med switched to a biosimilar last month. Was nervous as hell, but my flare-ups haven’t changed at all. My bill? Cut in half. Honestly, if your doctor says it’s safe, give it a shot. No magic, just science.
Also, huge props to the FDA for keeping this rigorous. Too many people think ‘biosimilar’ = ‘cheap knockoff.’ It’s not. It’s a masterpiece of biotech.
PS: My pharmacist even printed me a little comparison sheet. That’s the kind of care we need more of.