When a generic drug hits the market, you assume it works just like the brand-name version. But what if the people taking it aren’t the same people who tested it? That’s the real issue behind bioequivalence studies and why age and sex matter more than most people realize.
Why Bioequivalence Studies Used to Ignore Women and Older Adults
For decades, bioequivalence (BE) studies were done almost entirely on young, healthy men. The logic was simple: if you give the same person two versions of a drug - say, a generic and the original - and measure how much enters the bloodstream, you can tell if they’re the same. Men were chosen because they didn’t have hormonal cycles, didn’t get pregnant, and were easier to recruit. It wasn’t malicious - it was convenient. But here’s the problem: men and women don’t process drugs the same way. Women often have slower stomach emptying, different body fat percentages, and variations in liver enzymes that break down medications. Older adults have reduced kidney function, lower muscle mass, and changes in how their bodies absorb drugs. If you only test on 25-year-old men, you’re not seeing how the drug behaves in the people who actually use it - like women over 60 taking blood pressure meds or postmenopausal women on thyroid hormones.What the FDA Now Demands (2023 Update)
The U.S. Food and Drug Administration (FDA) changed the rules in its May 2023 draft guidance. If a drug is meant for both men and women, the study must include roughly equal numbers of each - close to a 50/50 split. No more excuses. If you want to test only men, you have to prove why. That’s a big shift from just a few years ago, when sponsors could get away with 80% male participants. For older adults, the FDA now says: if your drug is meant for people 60 and up - like those for osteoporosis or Alzheimer’s - you need to include participants in that age group. Or, if you don’t, you must explain why. That’s new. Before, you could test everything on 20-year-olds and assume it worked the same for everyone else. Now, regulators expect you to prove it.How the EMA and ANVISA Compare
The European Medicines Agency (EMA) still says subjects “could belong to either sex,” but doesn’t require balance. That means a study could technically enroll 90% men and still pass - as long as the results show no difference between the two drug versions. That’s a major difference from the FDA’s stance. Brazil’s ANVISA is stricter than both. They require exact 50/50 male-female splits, participants must be between 18 and 50, and they can’t smoke. Their BMI must fall within 18.5 to 30. It’s a tight box. But ANVISA’s rules reflect a growing global trend: if you’re making a drug for real people, your test group should look like them.
The Hidden Risk: False Bioinequivalence
Here’s something most people don’t know: small studies can lie. In one 2017 study with only 14 participants, a generic drug looked like it wasn’t bioequivalent in men - the blood levels were 20% lower. But in women, it was fine. At first, it looked like a safety issue. Then a follow-up study with 36 people showed the opposite: the difference vanished. What happened? In small groups, a few outliers can skew everything. One woman with unusually fast metabolism made the generic look bad. One man with slow absorption made it look worse. That’s why regulators now push for larger studies - at least 24 to 36 participants. More people mean the weird outliers balance out. It’s not about being perfect. It’s about avoiding false alarms.Why Recruitment Is Still a Mess
Even with clear rules, getting enough women and older adults into studies is hard. Sites report recruitment takes 40% longer when you need equal numbers of men and women. Why? Women are more likely to have caregiving duties, work non-standard hours, or be wary of clinical trials after past ethical abuses. Older adults may have multiple health conditions that disqualify them - even if they’re otherwise healthy. And cost? Recruiting women can add 20-30% to the price tag. Many sponsors still try to avoid it. But the FDA is watching. Between 2015 and 2020, only 38% of generic drug applications hit the 40-60% female participation range. The median? Just 32%. That’s not just bad science - it’s bad medicine.Real-World Consequences: When Studies Don’t Match Reality
Take levothyroxine, a drug used by millions for hypothyroidism. About 63% of users are women. But in most bioequivalence studies for this drug, fewer than 25% of participants are female. What happens when a woman switches to a generic version that was only tested on men? She might feel tired, gain weight, or get heart palpitations - not because the drug is bad, but because her body processes it differently. The same goes for antidepressants, statins, and blood thinners. Women metabolize many of these drugs slower. Older adults clear them even slower. If the study doesn’t reflect that, you’re setting people up for side effects or ineffective treatment.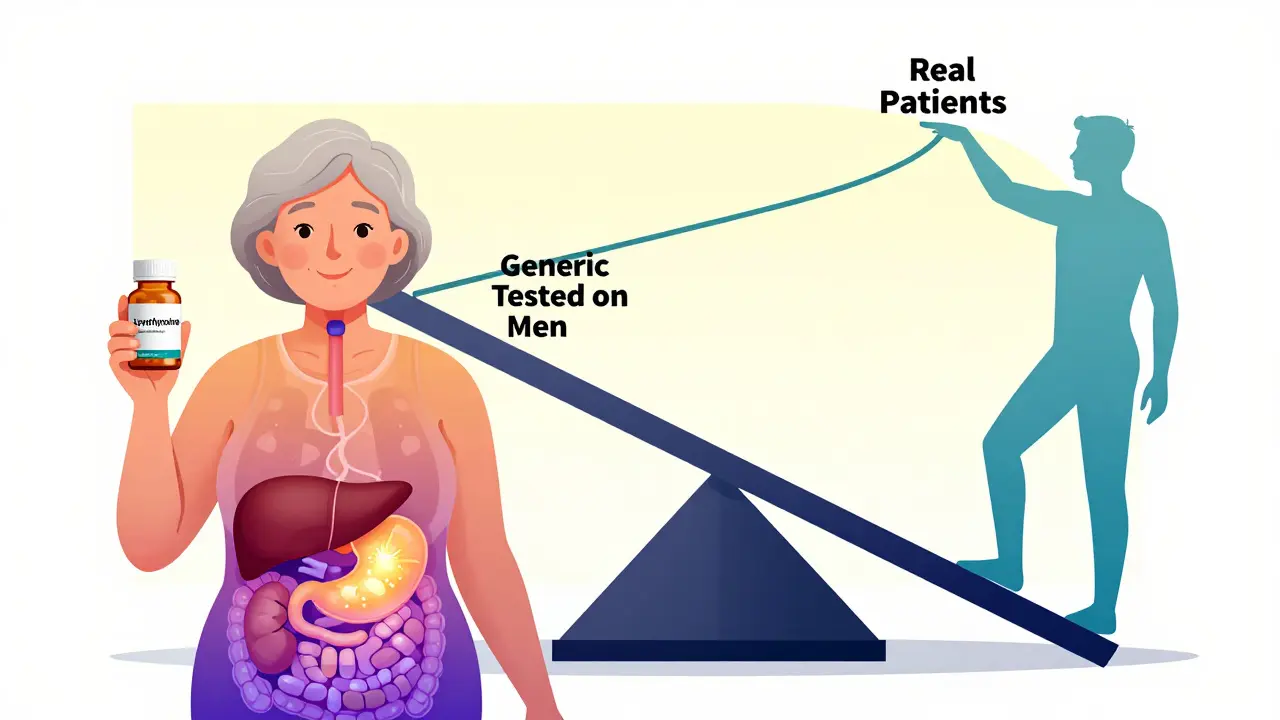
What’s Next? The Future of Bioequivalence
The EMA is reviewing its 2010 guidelines and may update them in 2024. The FDA’s 2023 draft is likely to become final soon. Both agencies are moving toward requiring sex-specific data analysis - not just counting men and women, but looking at whether the drug behaves differently in each group. New research from the University of Toronto in 2023 found that 37% of commonly tested drugs are cleared 15-22% faster in men than in women. That’s not a small difference. It could mean women need slightly higher doses, or longer dosing intervals. Right now, we don’t know because we don’t test it. The next step? Bioequivalence criteria for narrow therapeutic index drugs - like warfarin or lithium - may soon be sex-specific. That’s coming. And when it does, the old way of testing will be outdated.What Sponsors and Regulators Need to Do Now
If you’re developing a generic drug:- Don’t assume what works for young men works for everyone.
- Plan for balanced enrollment from day one - don’t wait until the last minute.
- Use stratified randomization: make sure each sex is evenly split across treatment groups.
- Pre-specify subgroup analyses in your protocol. Don’t just look at the whole group - check men and women separately.
- If you exclude older adults, document why. Is it safety? Feasibility? Justify it with data.
- Reject studies that don’t reflect the target population unless there’s strong justification.
- Require sex-specific pharmacokinetic data in Clinical Study Reports.
- Push for real-world data to supplement BE studies - especially for chronic conditions.
Final Thought: Bioequivalence Isn’t Just About Chemistry
A pill might have the same active ingredient, the same dosage, the same coating. But if it was only tested on one kind of body, it’s not truly equivalent for everyone. Bioequivalence isn’t just about math and blood samples. It’s about fairness. It’s about making sure the person who picks up a generic drug - whether they’re a 72-year-old woman or a 35-year-old man - gets the same result. That’s not just good science. It’s good medicine.Why are bioequivalence studies often done only on young men?
Historically, bioequivalence studies used young, healthy men because they had fewer variables - no hormonal fluctuations, no pregnancy risk, and were easier to recruit. This practice started in the 1980s and continued for decades despite evidence that sex and age affect drug metabolism. Regulatory agencies didn’t require diversity until recently, so sponsors followed the path of least resistance.
Does the FDA require equal numbers of men and women in bioequivalence studies?
Yes, according to the May 2023 draft guidance. If a drug is intended for both sexes, applicants must include similar proportions of males and females - typically close to a 50/50 split. Deviations require strong scientific justification. This is a major change from earlier guidelines, which only recommended balanced enrollment without requiring it.
What age groups should be included in bioequivalence studies?
The FDA requires participants to be at least 18 years old. For drugs intended for older adults - such as those for osteoporosis or dementia - studies must include participants aged 60 or older, or provide a clear scientific justification for excluding them. The EMA requires participants to be 18+, while ANVISA limits enrollment to 18-50 years. Age requirements vary by region, but the trend is toward including populations that will actually use the drug.
Can women participate in bioequivalence studies if they’re of childbearing potential?
Yes, but they must use effective contraception or practice complete abstinence during the study and for a specified period afterward. Pregnancy and lactation are strictly prohibited because of potential risks to the fetus or infant. The FDA specifically requires documented contraceptive use in its guidelines, and all major regulatory agencies follow similar rules.
Why do small bioequivalence studies sometimes show false sex differences?
Small studies - especially those with fewer than 20 participants - are vulnerable to outliers. One person with unusually high or low drug absorption can distort results, especially if they’re the only woman or only older adult in the group. Larger studies (24-36 participants) reduce this risk by spreading out variability across more people. This is why regulators now recommend minimum sample sizes and pre-specified subgroup analyses to avoid misleading conclusions.
Are there drugs where sex differences in bioequivalence are well-documented?
Yes. Studies have shown clear sex-based differences in the metabolism of drugs like statins (e.g., simvastatin), certain antidepressants (e.g., sertraline), and sedatives (e.g., zolpidem). For example, women often have higher blood levels of zolpidem, leading to increased risk of next-day drowsiness. The FDA now requires sex-specific dosing recommendations for some of these drugs, and bioequivalence studies must account for these patterns to avoid safety issues in generic versions.
What happens if a generic drug fails to meet bioequivalence standards in women?
If a generic drug shows significantly different absorption in women compared to the brand-name version, the FDA will not approve it - even if it passes in men. The goal is to ensure the generic works safely and effectively for everyone in the target population. In such cases, the sponsor must reformulate the drug, adjust the dose, or provide additional data to demonstrate consistent performance across sexes.
How do regulators ensure studies are properly designed for age and sex?
Regulators review the study protocol before the trial begins. They check inclusion/exclusion criteria, sample size justification, randomization methods, and planned subgroup analyses. Clinical Study Reports must include detailed demographic data, baseline characteristics, and sex- and age-specific pharmacokinetic results. If these are missing or inadequate, the application is put on hold until corrections are made.


Paula Villete on 24 December 2025, AT 11:25 AM
Finally. Someone’s talking about this like it matters. I’ve been on levothyroxine for 12 years and switched generics three times. Each time, I felt like a zombie for two weeks. My doctor shrugged and said, 'It’s the same drug.' But my body knew better. Turns out, the last generic was tested on 28 guys under 30. No wonder I couldn’t sleep, couldn’t think, and cried during commercials.
It’s not just chemistry-it’s compassion. And if regulators still act like women are just men with extra organs, we’re gonna keep paying the price in fatigue, weight gain, and lost productivity.
Also, why do we still call this 'bioequivalence' when it’s clearly 'bio-same-for-men-only'? Just say it. Be honest.